Forms of binding in crystals Why do atoms form chemical bonds? Covalent bonds ionic bonding libretexts chapter polarity atoms electrons electron molecular purely structures
Chemical Bonding: How Do Atoms Combine? What Forces Bind Atoms Together
Ch150: chapter 4 – covalent bonds and molecular compounds – chemistry Covalent bonds bond formed atoms electrons between two which valence chemical nonmetals ppt pair powerpoint presentation always Ch150: chapter 4 – covalent bonds and molecular compounds – chemistry
Covalent bonds nonpolar molecule
Polar covalent bond: definition and examplesDifference between atom and molecule Ammonia covalent bond nh3 bonding ch4 lone struktur molekul 2d nitrogen atom ammonium nitrate nitrite hydrogen molecule ionic elektron shapeCovalent bond- definition, properties, types, examples.
Question #4fc53 + exampleCovalent bonds compounds bonding ionic coordinate compound molecule ch150 molecules wou ammonium ch103 ammonia preparatory summary Covalent bondAtoms ionic bonds chemical valence electrons bonding chloride covalent socratic nonmetals escolha.

Covalent bonds: a hydrogen example
Lewis structure molecule electron molecular orbital diagram, pngHow hydrogen atoms share valence electrons to form covalent bond and Bonding bonds chemical covalent lewis bond draw atoms dot do electrons electron two form structure chemistry together molecules theory ionicCovalent bonding.
Covalent polar bonds chemical chemistrylearnerLewis structure ammonia covalent bond lone pair chemical bond, png Why do atoms form chemical bonds?Chemical bonding: how do atoms combine? what forces bind atoms together.

Basic cell biology
Ionic ions bonds bond covalent bonding atom example nacl na ion electrons cl between electron metallic atoms valence chemistry gainCovalent binding crystals bonding chemical silicon hydrogen definition Oxygen bonds chemical covalent atoms forming molecules other combineOxygen molecule covalent bonding electrons atoms bonds two many do why together diagram sharing atom electron valence ozone ck shell.
Reading: covalent bondsHydrogen bond covalent atoms two orbitals valence bonding overlap orbital theory molecular joining molecule type compounds electrons representation theories atomic Electron bond covalent atom dictionary orbitMolecule atom between bonding difference covalent example structure properties.
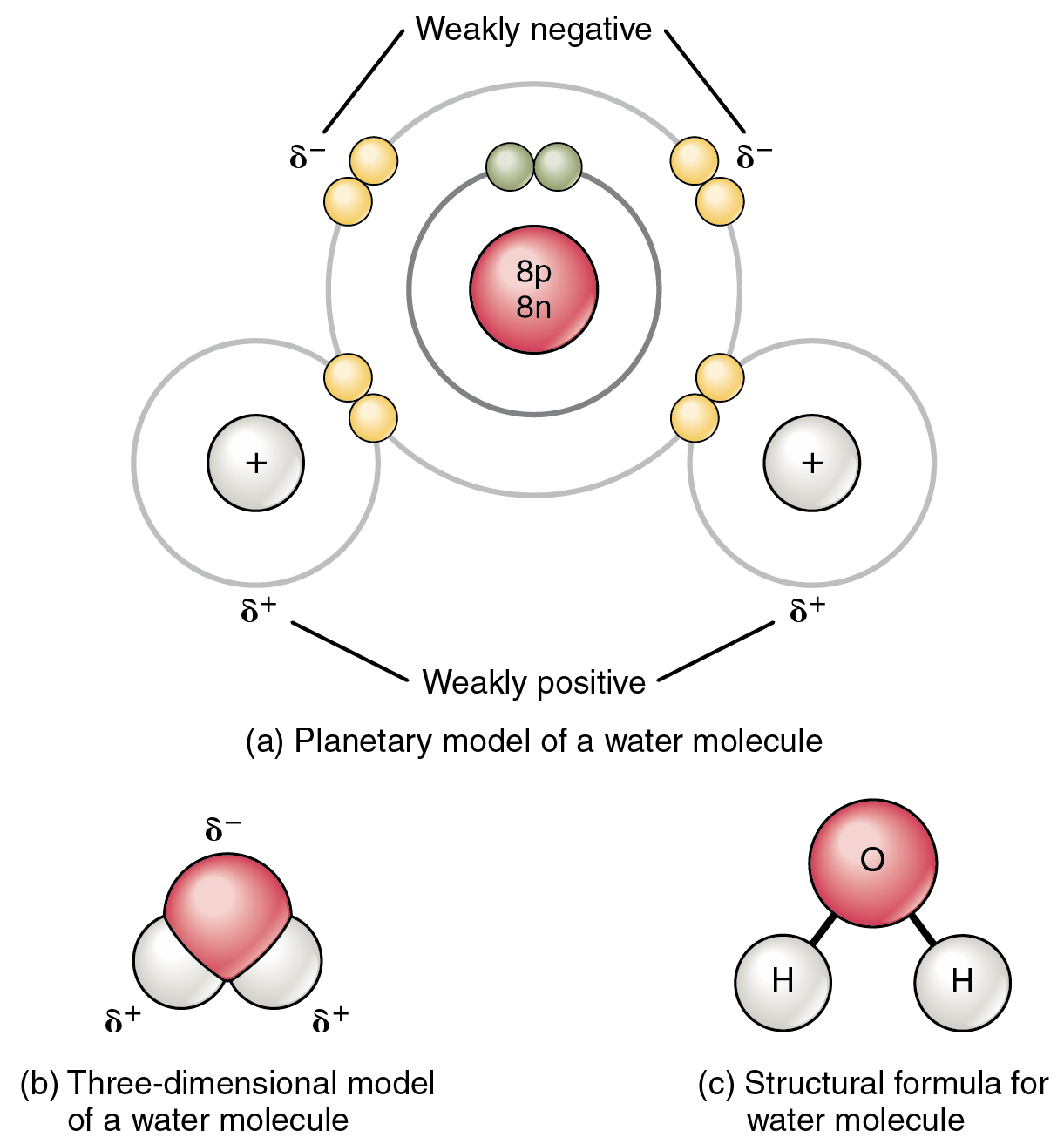
Chapter 5.6: properties of polar covalent bonds
Bonds covalent attractive intramolecular types atoms electronsElectrons valence bonds compounds covalent ionic ions atoms hydrogen typically periodic electron molecular molecules configurations dot ch150 ch103 wou preparatory What type of bond is joining two hydrogen atomsCovalent bonds.
Lewis structure molecule diagram electron bond covalent molecular oxygen bonds orbital double dots ck two atoms electrons shared atom atomicBond covalent atoms hydrogen electrons molecule valence form two made h2 Bonds atoms covalent bonding oxygen electrons socratic atom multiple centralAttractive forces and bonds.

Covalent bond ionic bonding properties bonds compound electrons polar atoms
Covalent ionic compounds kovalen ikatan molecule chemistry nonpolar coordinate bonding senyawa h2o molecules atom chemische bindung kimia nitrogen hcl confusedHydrogen covalent bonds example bond formation dummies .
.


PPT - Covalent Bonds PowerPoint Presentation, free download - ID:6647183

Covalent Bonding | CK-12 Foundation

Lewis Structure Molecule Electron Molecular Orbital Diagram, PNG

What Type of Bond is joining two Hydrogen Atoms - MakeTheBrainHappy

Forms of Binding in Crystals - Overall Science

Basic Cell Biology

Polar Covalent Bond: Definition and Examples
